VEC exhibitors
CORPORATE INFORMATION
Although the 22nd EFORT Annual Congress Vienna 2021 has been cancelled, we are pleased to leave here the links and information of our existing corporate partners and sponsors.
EFORT invites you to browse through the Guide of Corporate Partners and Sponsors of the VEChybrid 2021 which represents those EFORT clients that have committed to and confirmed their participation in the 2021 EFORT Annual Congress.
EFORT Industry Database
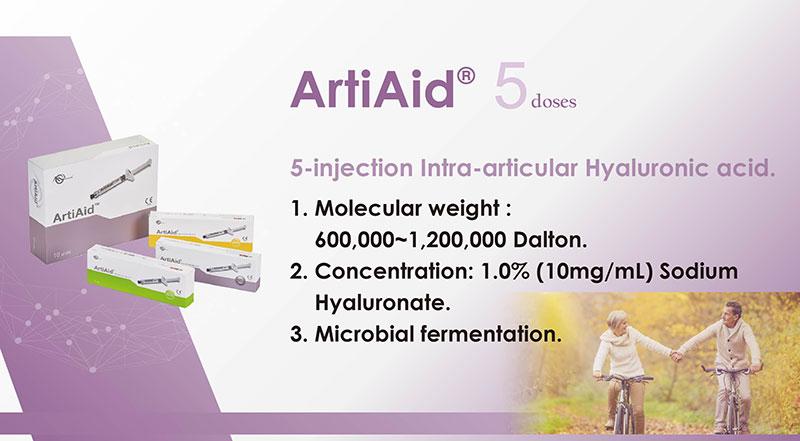
MAXIGEN BIOTECH INC.
Maxigen Biotech Inc. (MBI) was founded in December 1998. Next year, MBI stationed in National Yang-Ming University and set up an incubation center at there to conduct the R&D work of collagen. In June,2002, Global Investment Holdings made capital investment for MBI and became one of the shareholders. In the Decemeber of same year, MBI started building its own biomedical factory office building at Wugu industrial park. Next year, MBI set up the skin care product facility in Sigang township, Tainan county. MBI utilizes its core competence in biopolymers and has developed a broad range of applications in areas such as medical devices and skincare products. MBI specializes in the developing, manufacturing, and marketing of biomedical and skincare products. Headquarters was moved to Hwa-Ya Technologypark in July, 2016. Our major shareholders include GIH Group, Mega International Commercial Bank, First Financial Holding, Shanghai Fuson Pharmaceutical Group Company and – NuVasive Inc., Nasdaq-listed medical device company in the US.
MBI's Biomedical facility is certified by Taiwan GMP, ISO13485 and Korean KGMP. Furthermore, it passed US FDA inspection in 2009 and 2015 with no significant deviation being observed. MBI's biomedical products are mainly applied to the usage of orthopedics, aesthetic medicine, dental and ophthalmology. Production quality is in accordance with international regulations and requirements. Also our products have been certified by Taiwan, China, USA, Europe, Southeast Asia, Middle East and other emerging markets.
MBI's Skincare Product Division specializes in the design of various types of unique formulations and it has an ISO 22716 and GMP certified manufacturing facility. Skincare division provides the most effective and reliable cosmetic products to meet the needs of various skin types. We OEM for many well known global brands as well as Taiwan domestic ones.These products including various kinds of mask series, cleansing series, skin care series, hair care series and body care series. These Products are well received and marketed all over the world.
Benefit from the core competence of biotech division, skin care R&D center also has its own proprietary development capabilities, such as the development of platinum, gold particles conductive technology raw materials "double gold crystal peptide". This is combined with our own proprietary manufacturing know-how of collagen and hyaluronic acid, therefore we are capable of developing anti-aging wrinkle, low sensitivity, moisturizing, whitening and other innovative products series for the skin regeneration and repair with more efficacy. In addition, MBI also works closely with the internationally well-known institutions then to introduce new technology collaboration and commercialization, to make the skin young, elastic again.