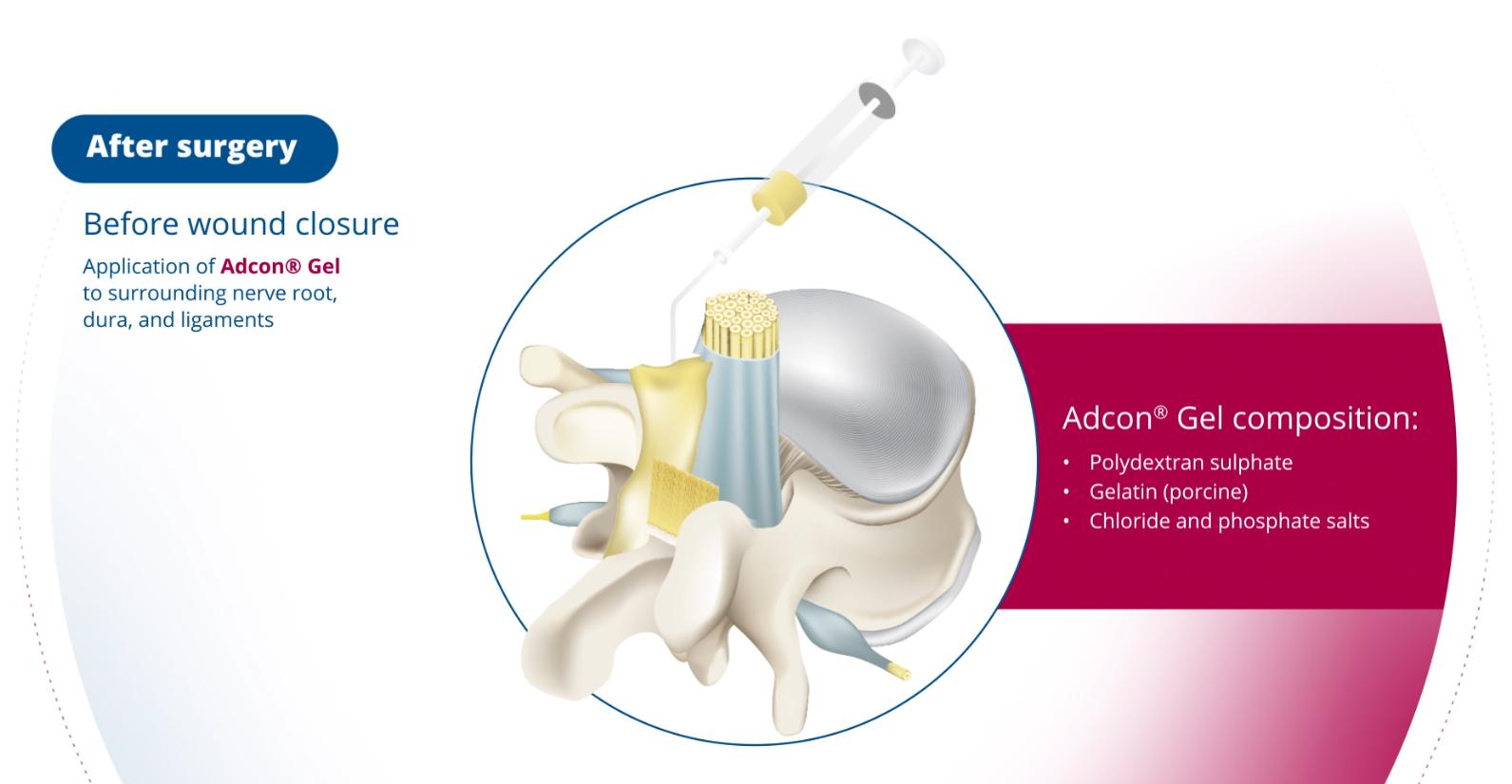
Adcon Gel | anti-adhesion gel
Adcon® Gel is a biocompatible, resorbable gel that provides a physical barrier to intertissue adhesions and inhibits fibroblast migration. This results in improved patient rehabilitation, reduced recurrent pain, and decreased number of re-operations as a result of such adhesions. Prevents adhesion formation: physical barrier to inter-tissue adhesions, adheres to the tissues it is applied onto, and inhibits fibroblast migration on and around neural tendinous structures Resorbable: biocompatible gel that gradually resorbed over a period of 4-6 weeks Adcon® Gel reduces and simplifies re-operations, resulting in lower incidence of Failed Back Surgery Syndrome (FBSS) and eliminates the need to perform differential diagnostics (X-rays, MRIs) Extensive clinical history: used in over 300 thousand cases worldwide and in more than 10 clinical studies since its introduction in 1996 Ease of use: special applicator for easy, yet secure application of the gel to difficult to reach or irregular structures
Category: Spine Intervention

C-ment and C-ment NXT PMMA bone cement
Leader Biomedical offers a comprehensive bone cement portfolio with C-ment® NXT bone cements, with different volumes and in different viscosities, as well as excellent handling properties in terms of performance and versatility that address specific user needs: from the experienced surgeons to the next generation of orthopaedic surgeons. Our facilities have the highest commitment concerning product quality, reliability and efficacy. Our C-ment® NXT variants are our Next-Gen cement for extra versatility and diverse user needs, and offer even longer, adaptable handling time, are more forgiving in preparation, and allow surgeons increased flexibility and enhanced ease of use during hip and knee implant surgeries.
Category: Joint Care Reconstruction
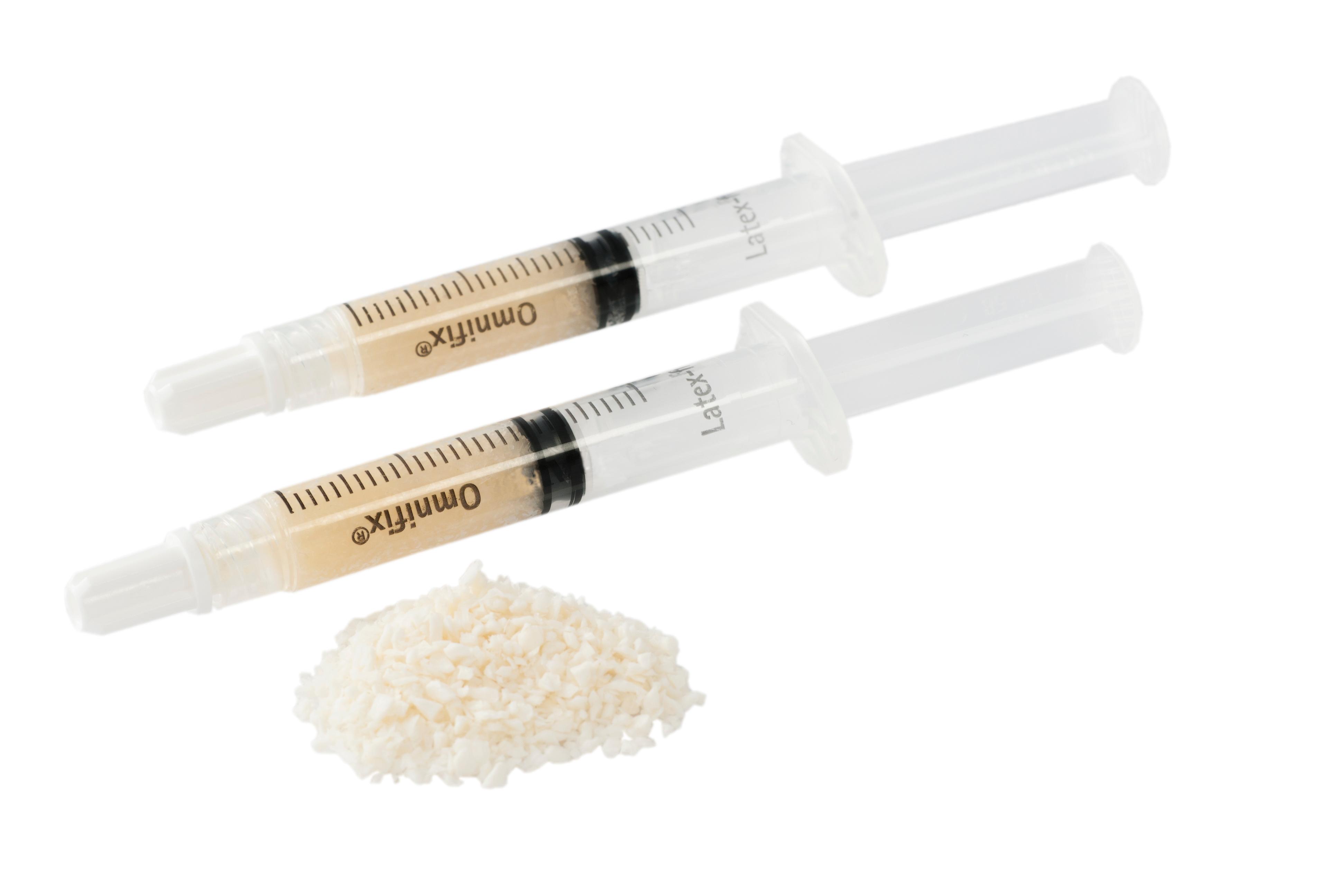
eTiss DBM| perfecting bone regeneration
eTiss® DBM Demineralised Bone Matrix Cortical bone demineralised with the goal of exposing encapsulated growth factors, such as bone morphogenetic proteins (BMPs). The presence of BMPs has proven to result in faster bone regeneration Available as putty in a pre-filled syringe for easy application Wide range of indications such as dental, spine, and joint care
Category: Joint Care Reconstruction

eTiss void fillers | perfecting bone regeneration
eTiss® void fillers Cancellous and cortical bone chips with sound structural and mechanical properties for filling of bone loss and/or correcting of defects. eCOO® Technology processing ensures excellent osteoconductive properties Forms stable complex with patient’s adjacent bone Wide variety of general orthopaedic, spinal, and dental applications
Category: Joint Care Reconstruction
Hyalosyn |Hyaluronic acid injections for all synovial joints to treat osteoarthritis
Hyalosyn™ Gel is a 1% sodium hyaluronate viscoelastic sterile solution, manufactured by synthetic fermentation, with no microbiological residues. Hyalosyn™ Gel comes in a re-filled syringe for easy injection into all synovial joints. Treatment with HA is clinically proven for osteoarthritis for over 10 years to facilitate improved joint mobility and reduce pain caused by osteoarthritis, or at first signs of movement limitations and joint pain. Manufactured by synthetic fermentation No microbiological residues Suitable for intra-articular injection into all synovial joints Sterile solution in pre-filled syringe with cap Easy injection
Category: Joint Care Intervention
Ossfinity | Synthetic bone substitute putty for guided bone and tissue regeneration
Leader Biomedical’s OssGro® and Ossfinity® line of synthetic bone grafts are produced with MBCP™ Technology, a proprietary method for producing high quality synthetic bone grafts composed of 60% HA and 40% βTCP. Unlike other bulk production methodologies, this process relies on a sophisticated process of synthesis with several heating steps. Ossfinity® combines OssGro® granules and an absorbable hydrogel to create a putty, which acts as a carrier for rapid vascularisation and mineralisation.
Category: Joint Care Reconstruction

OssGro | Synthetic bone substitutes for guided bone and tissue regeneration
OssGro® granules, sticks, and wedges are synthetic bone grafts produced using MBCP™ Technology, a proprietary method for producing high quality synthetic bone grafts composed of 60% HA and 40% βTCP. Unlike other bulk production methodologies, this process relies on a sophisticated process of synthesis with several heating steps. The use of this technology ensures good mechanical strength with resorption rates resembling real bone. New bone formation promoted by the release of calcium and phosphate ions Safe and easy to use Effective absorption of osteogenic proteins Long term scaffold effect for bone growth
Category: Joint Care Reconstruction
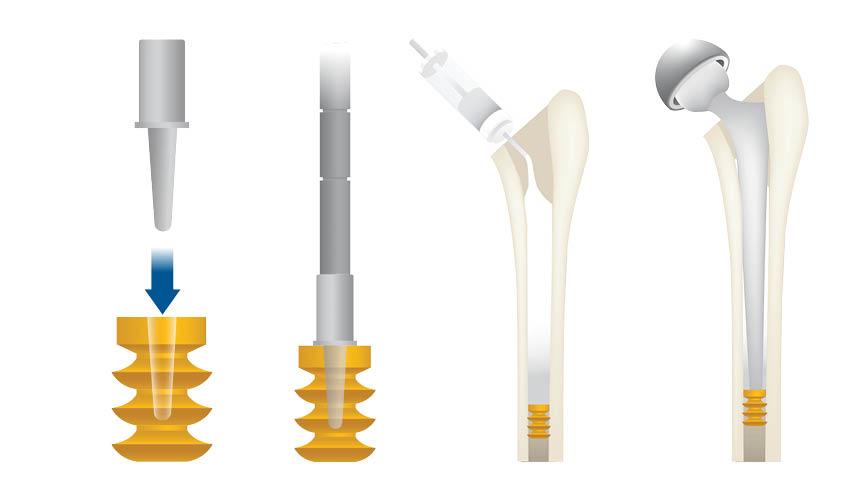
VarioPlug cement restrictor
VarioPlug® is a resorbable, biodegradable cement restrictor for orthopaedic use. It is designed to occlude the medullary cavity before the introduction of bone cement. VarioPlug® prevents cement penetration in the diaphysis, facilitates cement pressurisation during implantation of prosthesis and fully resorbs within a few days. Reduced distal pressure Resorbable Biodegradable Easy to use VarioPlug® is available in a wide range of sizes
Category: Joint Care Reconstruction
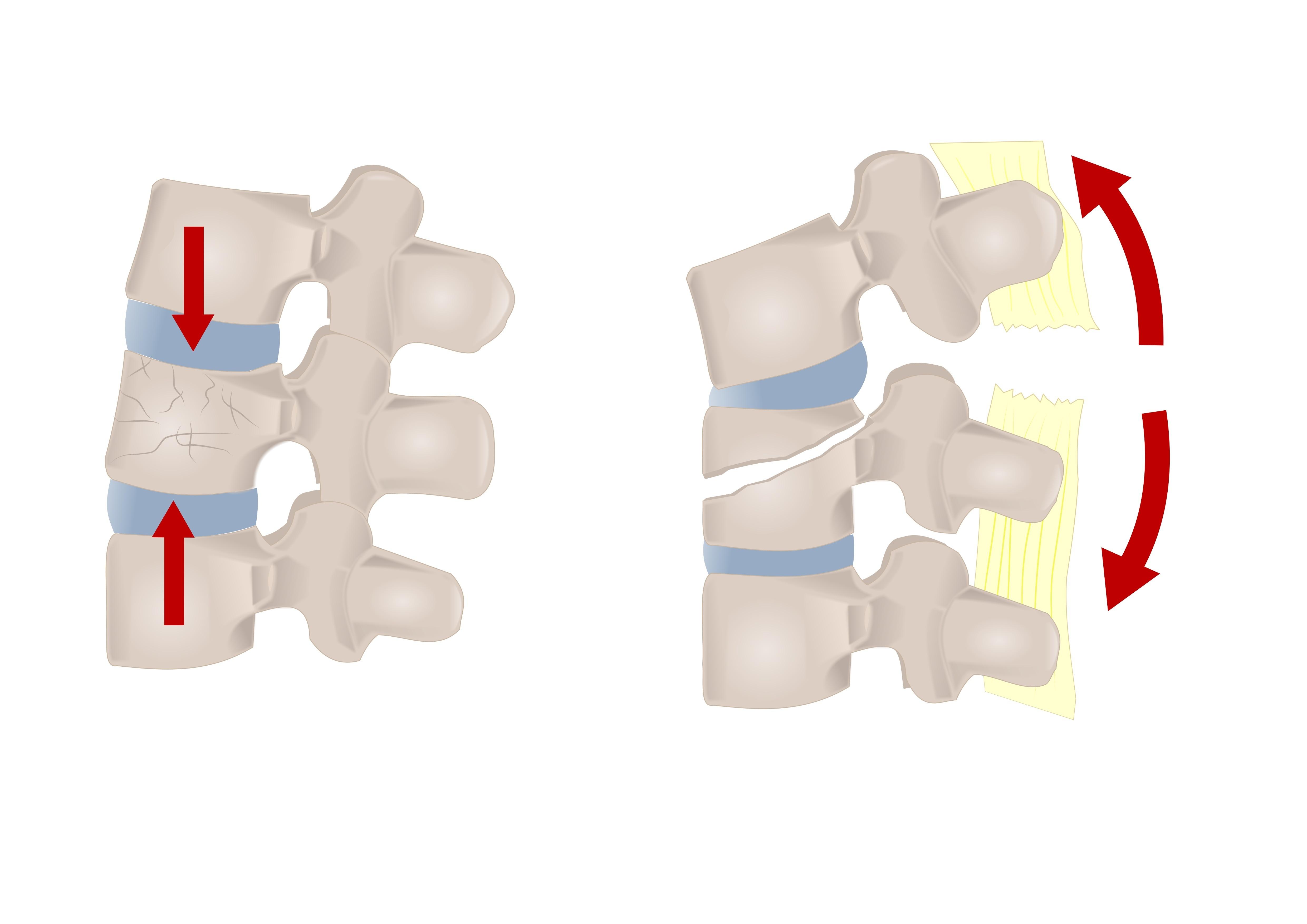
Vebroplast | Radiopaque vertebroplasty spine cement
Bone cement plays a critical role in the effectiveness of vertebroplasty treatments. The ideal cement must: Present significant strength and toughness to ensure long-term viability Present optimal viscosity to allow it to be injectable Have appropriate working and setting times to allow the correct pace of the procedure Be radiopaque and display clear contrast under fluoroscopy Vebroplast® is PMMA radiopaque spine cement used for strengthening, fixation, and filling of the vertebrae following vertebral compression fractures.
Category: Spine Stabilisation








